SINGAPORE, July 25, 2014 (ENS) – Australian and Taiwanese scientists have discovered a new molecule that may help resolve one of the barriers to development of hydrogen fuel cell cars – how to safely fuel up with the highly explosive gas.
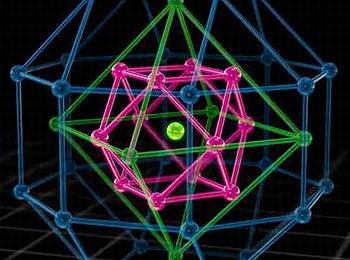
The new molecule, nicknamed the Chinese Puzzle Molecule, is a 28copper15hydride core wrapped in dithiocarbamate.
Scientists say that the new molecule puts us on a path to better understanding hydrogen, how to get it in and out of a vehicle’s fuel cell system, and how to store it in a stable, safe manner.

Several automakers are poised to bring hydrogen fuel cell cars to a wider market next year, including: the Toyota FCV, the Chevrolet Equinox Fuel Cell, Honda FCX Clarity, Hyundai is35 Fuel Cell and the Mercedes-Benz B-Class F-Cell.
A fuel cell separates the single electron in a hydrogen atom from the single proton and uses the electrons to produce a stream of electricity that can then power the motor in an electric vehicle.
But in the presence of oxygen, hydrogen can catch fire and explode, and it burns more easily than gasoline. Hydrogen requires only one tenth as much energy to ignite as gasoline does. A spark of static electricity from a person’s finger is enough to set it off.
Ideally, no oxygen should be present in the liquid hydrogen tanks in a fuel cell vehicle, but trace amounts of air may contaminate the hydrogen supply. If the hydrogen should escape, it will come into contact with the oxygen in air.
“28copper15hydride” is not a name developed by a marketing guru, but it has some of the world’s most savvy chemists intrigued.
Details were presented Thursday by Australia’s Dr. Alison Edwards at the 41st International Conference on Coordination Chemistry, Singapore, where 1,100 chemists have gathered.
The molecule was synthesized by a team led by Professor Chenwei Liu from the National Dong Hwa University in Taiwan.
The chemical structure determination was completed by the team at the Australian Nuclear Science and Technology Organisation (ANSTO) using the crystallography tool KOALA.

Most solid material is made of crystalline structures. The crystals are made up of regular arrangements of atoms stacked up like boxes in a tightly packed warehouse. The science of finding this arrangement, and structure of matter at the atomic level, is crystallography. ANSTO is Australia’s home of this science.
ANSTO’s Dr. Edwards, a chemical crystallographer at the Bragg Institute, explains the elementary principles behind the discovery, and the discovery itself.
“Anyone with a textbook understanding of chemistry knows the term ‘hydride’ describes a compound which results when a hydrogen atom with a negative charge is combined with another element in the periodic table,” said Edwards.
“This study revealed that mixing certain copper (Cu) compounds with a hydride of boron (borohydride or (BH4)) – created our newly discovered “Chinese Puzzle molecule” with a new structure that has alternating layers of hydride and copper wrapped in an outer shell of protecting molecules,” she said.
“Using our leading KOALA instrument – we identified that this molecule actually contained no less than 15 hydrides in the core – which is almost double the eight we were expecting.”
“This new molecule has an unprecedented metal hydride core – it is definitely different and much more stable than many previous hydride compounds, in fact it is stable in air, which many others are not,” Edwards said. “So, we see there is probably much more yet to learn about the properties, and potential of hydride.”
The discovery puts us one step further along a path to developing distribution infrastructure – one of four obstacles to hydrogen fuel-cell technology as a viable power source for low-carbon motor vehicles.
Edwards says ANSTO’s KOALA is uniquely well placed for gaining a scientific understanding of hydrogen and the potential of hydrides, because “the neutron source allows us to see the precise location of hydrogen in structures even if it is invisible under X-rays.”
You cannot have a well-founded hydrogen economy, said Edwards, “unless you understand hydrogen.”
“No one is claiming hydrogen-powered cars are imminent,” she said. “Perhaps this puts us a step further down the road, but we don’t know how long the road is. What this research shows is hydrides may yet help us get hydrogen in and out of a fuel system, stored in a manner which is stable and safe – overcoming the Hindenburg-type risks.”
The Hindenburg disaster took place on May 6, 1937, as the German passenger airship LZ 129 Hindenburg, filled with hydrogen for buoyancy, caught fire and was destroyed during its attempt to dock with its mooring mast at the Lakehurst Naval Air Station, New Jersey.
“The implications from the research are actually broader and have impacts beyond car power sources,” Edwards said. “Through understanding the process, we have the prospect of controlling and even directing it.”
Copyright Environment News Service (ENS) 2014. All rights reserved.
