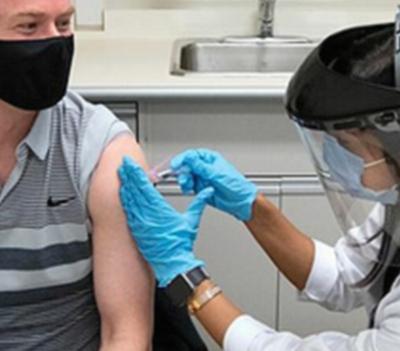
NEW YORK, New York, November 11, 2020 (ENS) – Pharmaceutical giant Pfizer is the first company to announce a successful vaccine candidate that is safe and effective in preventing COVID-19, the disease caused by the novel coronavirus called SARS-CoV-2.
Pfizer Chairman and CEO Albert Bourla Monday announced positive efficacy results from the company’s Phase 3, late-stage study of its potential COVID-19 vaccine. “The vaccine candidate was found to be more than 90 percent effective in preventing COVID-19 in participants without evidence of prior SARS-CoV-2 infection in the first interim efficacy analysis,” Boula said.
The apparently successful Pfizer study enrolled 43,538 participants, with 42 percent having racially and ethnically diverse backgrounds. One group of participants were given the vaccine candidate, known as BNT162b2, and the others were given a placebo.

The vaccine candidate has been shown to be more than 90 percent effective on these study participants, and no serious safety concerns have been observed, the company said in a statement. Safety and additional efficacy data continue to be collected.
The BNT162b2 vaccine candidate is not currently approved for distribution anywhere in the world. Pfizer says it plans to submit the vaccine candidate for Emergency Use Authorization to the U.S. Food and Drug Administration planned for soon after the required safety milestone is achieved, which the company expects to occur in the third week of November
But the European Union is not waiting for U.S. emergency authorization to order the vaccine.
Pfizer and the German immunotherapy company Biopharmaceutical New Technologies, BioNTech, today announced that they have reached an agreement with the European Commission to supply 200 million doses of their investigational BNT162b2 mRNA-based vaccine candidate gainst SARS-CoV-2 to EU Member States. The European Commission has the option to request an additional 100 million doses.
Vaccine doses for Europe will be produced in BioNTech’s German manufacturing sites, as well as in Pfizer’s manufacturing site in Belgium. If the BNT162b2 vaccine candidate receives approval from the European Medicines Agency, then doses will be ordered by the EU Member States who have elected to receive the vaccine as part of this agreement.
Deliveries are anticipated to start by the end of 2020, subject to clinical success and European regulatory authorization.
“Since the onset of the pandemic, Pfizer’s priority has been to develop a safe and effective vaccine, while simultaneously scaling up our manufacturing to deliver doses before the end of the year. This is an ambitious goal but critical to halting this global pandemic,” Bourla said. “Today’s finalized supply agreement with the European Commission represents the largest initial order of vaccine doses for Pfizer and BioNTech to date and a major step toward our shared goal of making a COVID-19 vaccine available to vulnerable populations.”
The U.S. government announced Wednesday it has reached a deal to gain access to at least 100 million doses of a Pfizer coronavirus vaccine candidate if it proves to be safe and effective.
The Department of Health and Human Services and the Department of Defense announced the agreement with Pfizer for the large-scale production of over 100 million doses following the vaccine’s successful manufacture. Upon receipt of the first 100 million doses, the U.S. will pay the drugmaker $1.95 billion.
Assuming positive data and availability of the necessary safety and manufacturing data, and based on current projections, Pfizer and BioNTech expect to produce globally up to 50 million vaccine doses in 2020 and up to 1.3 billion doses in 2021. To meet those anticipated quantities and milestones, the companies have produced sufficient supply for their Phase 2/3 clinical trial and have begun to produce and stockpile their pandemic supply.
“As a company founded in the heart of Europe, we are looking forward to supplying millions of people upon regulatory approval,” said CEO and Co-founder of BioNTech Ugur Sahin, M.D. “We would like to thank the Commission and the Member States for their support and trust in our COVID-19 vaccine candidate. Our aim is to develop a safe and effective vaccine to contribute to bringing this pandemic to an end. Only through joint efforts will we be able to do so.”
In a year that has seen more than 1.27 million lives lost to the coronavirus pandemic, WHO’s 194 Member States are expected to adopt a resolution to strengthen preparedness for health emergencies at the resumed 73rd World Health Assembly now underway in Geneva.
WHO Director-General Dr. Tedros Adhanom Ghebreyesus warned the audience at the opening of the Assembly on Monday that “a vaccine cannot address the global under-investment in essential public health functions and resilient health systems, nor the urgent need for a “One Health” approach that encompasses the health of humans, animals and the planet we share. There is no vaccine for poverty, hunger, climate change or inequality.”
He called for “leadership built on mutual trust and mutual accountability – to end the pandemic and address the fundamental inequalities that lie at the root of so many of the world’s problems.”
“It’s time for the world to heal from the ravages of this pandemic, and the geopolitical divisions that only drive us further into the chasm of an unhealthier, un-safer and unfairer future”Dr. Tedros said. “Today and every day, we must choose health. We’re one big family.”
Globally, as of 5:52pm CET, November 11, 2020, there have been 51,251,715 confirmed cases of COVID-19, including 1,270,930 deaths, reported to the World Health Organization.



