BERKELEY, California, September 9, 2015 (ENS) – Scientists have learned how to produce liquid fuel by creating artificial plants that make gasoline and natural gas using only sunlight – a process called synthetic photosynthesis.
These fuels can be used to run cars or heat buildings without emitting any greenhouse gases.
Peidong Yang, a professor of chemistry at the University of California, Berkeley and co-director of the school’s Kavli Energy NanoSciences Institute, leads a team that has created an artificial leaf.
The leaf produces methane, the primary component of natural gas.
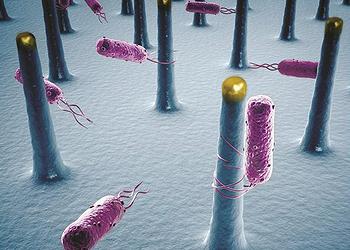
To accomplish this, Yang and his colleagues used a combination of semiconducting nanowires and bacteria, according to their paper describing the research in the online edition of the journal “Proceedings of the National Academy of Sciences” in August.
The process leads to synthetic photosynthesis, a type of solar power based on the ability of plants to transform sunlight, carbon dioxide and water into sugars.
Instead of sugars, synthetic photosynthesis seeks to produce liquid fuels that can be stored for months or years and distributed through existing energy infrastructure.

The scientists’ first system employs long nanoscale filaments called nanowires to turn sunlight into electrons, which bacteria use to convert carbon dioxide, CO2, and water into complex chemicals.
In the second system, nanowires generate electricity that splits water (H2O) into hydrogen and oxygen. Bacteria then combine the hydrogen with CO2 to form methane, the largest component of natural gas.
In a roundtable talk with his colleagues held by The Kavli Foundation, Yang explained, “One purpose of this experiment was to show we could integrate bacterial catalysts with semiconductor technology. This lets us understand and optimize a truly synthetic photosynthesis system.”
Yang was joined at the roundtable by Dr. Thomas Moore, a professor of chemistry and biochemistry and past director of the Center for Bioenergy & Photosynthesis at Arizona State University. He is a past president of the American Society for Photobiology, and a team leader at the Center for Bio-Inspired Solar Fuel Production.
Moore explained the need for the team’s artificial plants.
“In order to create sustainable, solar-driven societies, we need a way to store solar energy. With solar cells, we can make electricity efficiently, but we cannot conveniently store that electricity to use when it is cloudy or at night,” he said.

“If we want to stockpile large quantities of energy, we have to store it as chemical energy, the way it is locked up in coal, oil, natural gas, hydrogen and biomass,” Moore said.
Yang agreed. “Perhaps, one day, researchers will come up with an effective battery to store photoelectric energy produced by solar cells. But photosynthesis can solve the energy conversion and storage problem in one step. It converts and stores solar energy in the chemical bonds of organic molecules.”
The third roundtable participant was Dr. Ted Sargent, professor of electrical and computer engineering at the University of Toronto where he is vice-dean for research for the Faculty of Applied Science and Engineering. Sargent holds the Canada Research Chair in nanotechnology and is a founder of two companies, InVisage Technologies and Xagenic.
“Much of the globe’s power infrastructure, from automobiles, trucks and planes to gas-fired electrical generators, is built upon carbon-based fossil fuels,” said Sargent. “So creating a new technology that can generate liquid fuels that can use this infrastructure is a very powerful competitive advantage for a renewable energy technology.”
Also, our energy needs change with the seasons. Here in Canada, heating drives up energy use in winter. Maybe we could build a battery to store enough energy to heat our homes overnight, but the greater long-term challenge is to store energy we capture in the summer and use it to heat our nation of 35 million people in the winter.
The remarkable energy density of fossil fuels, all of which store energy created by ancient photosynthesis, make this possible. So while converting sunlight to fuels will always have a greater energy cost than making electricity, liquid fuels have a notably higher value because they can meet seasonal gaps between the supply and demand of renewables.
“Synthetic photosynthesis is a carbon-neutral solution,” said Sargent, “because we take one CO2 molecule out of the atmosphere for every CO2 molecule that we return during combustion.”
Yang concluded, “To come up with better synthetic catalysts, we need to learn from nature on the atomic and molecular scale. So it’s very important for researchers from different research communities to come together, talk to each other, and exchange ideas.”
PHOTO: Dr. Peidong Yang at the University of California, Berkeley (Photo courtesy The Kavli Foundation)
© 2015, Environment News Service. All rights reserved. Content may be quoted only with proper attribution and a direct link to the original article. Full reproduction is prohibited.
