RICHLAND, Washington, August 18, 2021 (ENS) – Your ride might be a modest one, but if it burns gasoline, you have precious metals on board. To reduce pollution at the tailpipe, gasoline cars and trucks today come equipped with catalytic converters that contain platinum-group metals such as rhodium and palladium.
Demand for these metals is mounting as countries around the world seek to lower vehicle emissions that accelerate climate change and worsen air quality.
Given that a single ounce of rhodium now costs more than $20,000, it’s no coincidence that across the United States, thefts of catalytic converters are on the rise.
A discovery by scientists from the Pacific Northwest National Laboratory, PNNL, and Washington State University could help reduce the amount of expensive metals needed to treat vehicle exhaust by making the most of every precious atom.
The researchers demonstrated success in reducing carbon monoxide and nitrogen oxide emissions using at least three times less rhodium than a typical catalyst.
Their study is published in the journal “Angewandte Chemie International Edition” under the title, “Economizing on Precious Metals in Three-Way Catalysts: Thermally Stable and Highly Active Single-Atom Rhodium on Ceria for NO Abatement under Dry and Industrially Relevant Conditions.”
“What we reported here is contradictory to the conventional wisdom that you need rhodium atoms adjacent to each other, in the form of a nanoparticle, to do this chemistry,” said Yong Wang, a professor of chemical engineering at Washington State University who holds a joint appointment at PNNL.
The new research revealed that a simple change to that idea does the job even better.
Wang said, “We found that a single atom of rhodium can do an even better job of converting pollutants than a rhodium nanoparticle.”
Converting the Conventional
The work at PNNL relates to three-way catalysts, named for their ability to reduce carbon monoxide, nitrogen oxide, and hydrocarbons such as methane.
Nitrogen oxide is one of a set of pollutants known as NOx, components of smog that also indirectly contribute to atmospheric warming. Elevated levels of nitrogen dioxide (NO2), for instance, can damage the human respiratory tract and increase severity of respiratory infections and asthma.
Carbon monoxide (CO), a colorless, odorless, tasteless gas, in high concentrations is toxic to humans.

Within a gasoline vehicle’s catalytic converter, the three-way catalysts intercept and dismantle such chemical compounds before they reach the tailpipe. A three-way catalyst will convert NOx into nitrogen and carbon monoxide and hydrocarbons into carbon dioxide.
Aftertreatment systems based on such catalysts have been used for decades with internal combustion engines.
But now, in addition to the skyrocketing prices for the precious metals needed to build these systems, another issue threatens to lower their efficacy.
As vehicles become more fuel-efficient, the exhaust isn’t as hot. That is a problem for conventional catalysts that were designed to work within the high temperatures of older engines. They simply don’t work as well at lower temperatures.
“We found that a single atom of rhodium can do an even better job of converting pollutants than a rhodium nanoparticle.” – Yong Wang, Joint Appointee at PNNL
The U.S. Department of Energy, DOE, has partnered with domestic automotive manufacturers to meet the challenge of designing materials that can convert 90 percent of tailpipe emissions at 150 °C (302 °F), which is considered “low temperature” in the world of emissions control. Such materials must also be stable enough to hold up over miles and miles of travel.
Isolating Atoms
The new PNNL study builds on earlier work from Wang and colleagues where they “trapped” single atoms of platinum on a support.
The newer study took this same atom-trapping approach with rhodium. Catalysts with only 0.1 percent weight of atomically dispersed rhodium under model conditions met the DOE 150 °C challenge, converting 100 percent of nitrogen oxide at temperatures as low as 120 °C.
“Buried in the scientific literature, there are reports from the 1970s showing that isolated rhodium atoms could perform this reaction, but those experiments were done in solutions, and the rhodium atoms were hydrothermally unstable,” said Konstantin Khivantsev and Janos Szanyi, PNNL researchers who led the study with Wang.
“What inspired us was this new approach to doing atom-trapping at high temperatures. With that, we were able to show for the first time that single rhodium atoms could be both catalytically active and stable,” they said.
Institute for Integrated Catalysis, Pacific Northwest National Laboratory (Photo courtesy PNNL)
The researchers conducted experiments for the study at the Environmental Molecular Sciences Laboratory, EMSL, a national scientific user facility sponsored by the U.S. Department of Energy’s Biological and Environmental Research program. It is co-located with the Pacific Northwest National Laboratory in Richland.
To verify that the rhodium atoms were dispersed individually and reacting effectively with carbon monoxide and nitrogen oxide to convert NOx into nitrogen and carbon monoxide and hydrocarbons into carbon dioxide, the scientists had some of the latest technology at hand.
At EMSL, they used various types of high-resolution imaging, including Fourier-transform infrared spectroscopy, transmission electron microscopy, and energy dispersive X-ray spectroscopy.
Khivantsev, Szanyi, and Wang worked with other scientists on this research; co-authors are Carlos Garcia Vargas, Jinshu Tian, Libor Kovarik, and Nicholas Jaegers.
The scientists said their findings clear a pathway to make cost-effective, stable, and low-temperature catalysts that use rhodium far more efficiently than current ones.
The group is interested in extending the method that worked so well with rhodium to other, less-expensive, catalytic metals such as palladium and ruthenium to make removing pollutants from the exhaust of gas-burning vehicles cheaper and more efficient.
— By Christina Nunez
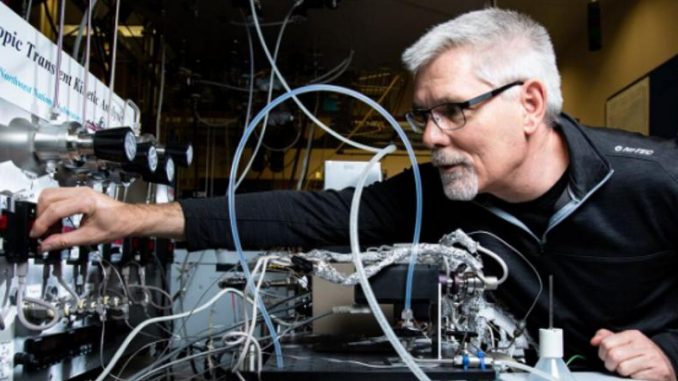
Featured image: Pacific Northwest National Lab researcher tests catalytic reactions on a Isotopic Transient Kinetic Analyzer (Photo courtesy PNNL)



